Publications
Also on Google scholar
† indicates equal contribution
‡ indicates corresponding authorship
Click on the graphical abstract to access the publication
Primary research articles
- Y. Zhang, A. Vinogradov, and H. Suga.‡ Ribosomal Synthesis of Topologically Defined Thioisoindole-Bridged Bicyclic Peptides. Angew. Chem. Int. Ed. 2025, e17689

- D. Nguyen, J. Ramos-Figueroa, A. Vinogradov, Y. Goto, M. Gadgil, R. Splain, H. Suga, W. van der Donk,‡ and D. Mitchell‡. Aminoacyl-tRNA specificity of a ligase catalyzing non-ribosomal peptide extension. J. Am. Chem. Soc. 2025, 147, 37893–37898.
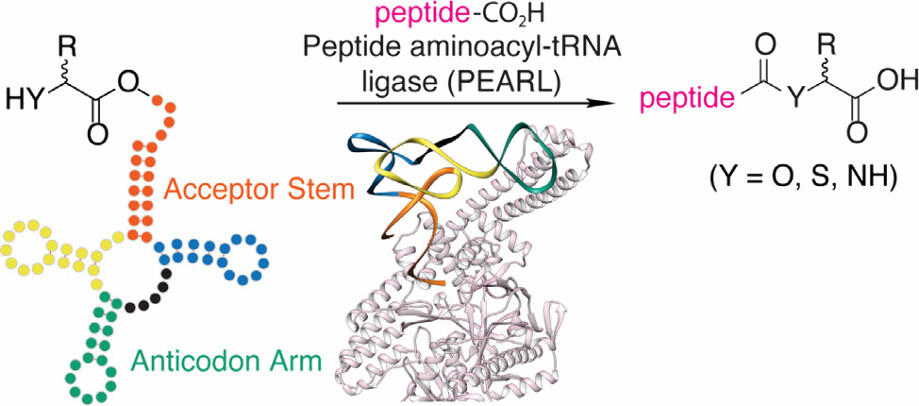
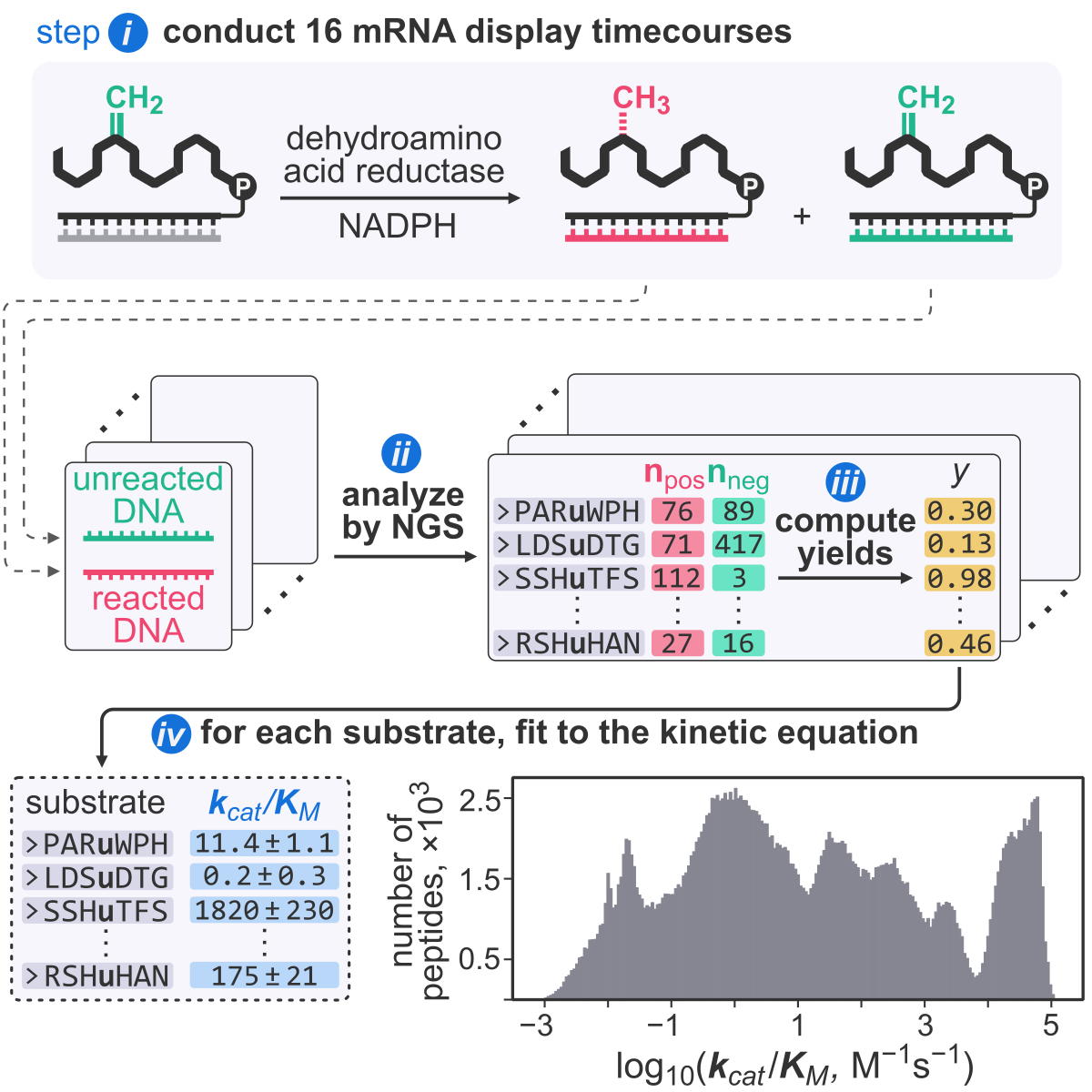
- A. Vinogradov‡ and H. Suga‡. Measuring kcat/KM values for over 200,000 enzymatic substrates with mRNA display. Chem, 2026, 12, 102737
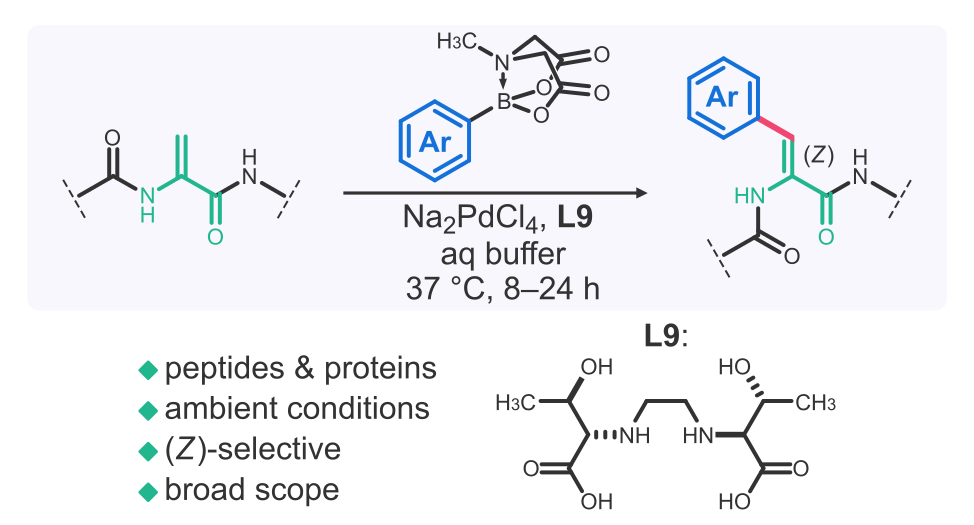
- A. Vinogradov‡, S. Pan, and H. Suga‡. Ligand-enabled selective coupling of MIDA boronates to dehydroalanine-containing peptides and proteins. J. Am. Chem. Soc. 2025, 147, 7533–7544
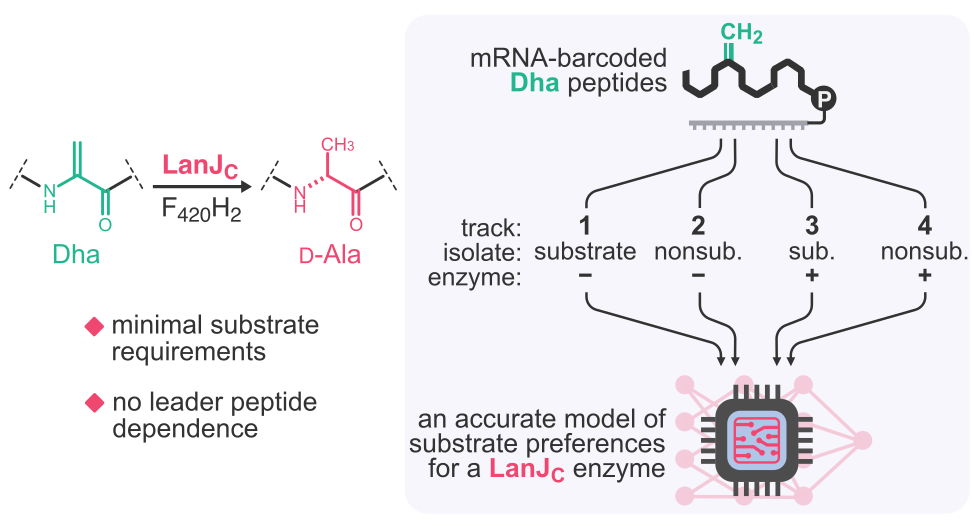
- A. Vinogradov,‡ G. Bashiri, and H. Suga‡. Illuminating substrate preferences of promiscuous F420H2-dependent dehydroamino acid reductases with 4-track messenger RNA display. J. Am. Chem. Soc. 2024, 146, 31124–31136.
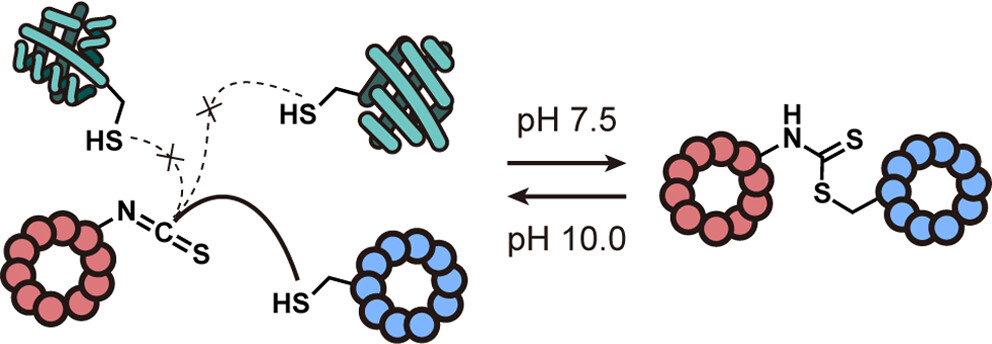
- Y. Ohno, A. Vinogradov,‡ and H. Suga‡. Selective pH-responsive conjugation between a pair of de novo discovered peptides. J. Am. Chem. Soc. 2024, 146, 29429–29440.
- H. King, M. Bycroft, T. Nguyen, G. Kelly, A .Vinogradov, P. Rowling, K. Stott, D. Ascher, H. Suga, L. Itzhaki, K. Artavanis-Tsakonas‡. Targeting the Plasmodium falciparum UCHL3 ubiquitin hydrolase using chemically constrained peptides. Proc. Natl. Acad. Sci. U. S. A., 2024, 121, e2322923121.

- A. Vinogradov,†,‡ Y. Zhang,† K. Hamada, S. Kobayashi, K. Ogata, T. Sengoku, Y. Goto,‡ and H. Suga‡. A Compact Reprogrammed Genetic Code for de Novo Discovery of Proteolytically Stable Thiopeptides. J. Am. Chem. Soc. 2024, 146, 8058−8070.
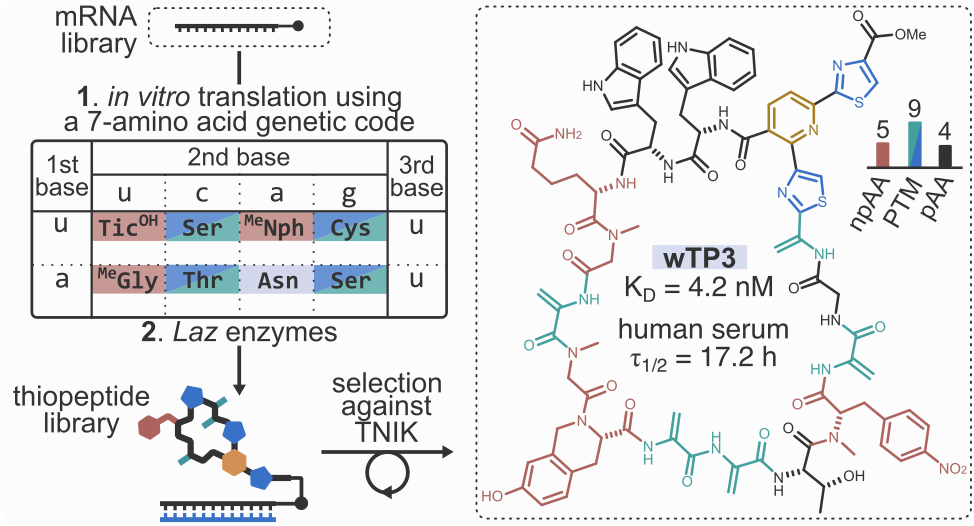
- J. Chang, A. Vinogradov,‡ Y. Zhang, Y. Goto,‡ and H. Suga.‡ Deep Learning-Driven Library Design for the De Novo Discovery of Bioactive Thiopeptides. ACS Cent. Sci. 2023, 9, 2150−2160.

- A. Vinogradov, Y. Zhang, K. Hamada, J. Chang, C. Okada, H. Nishimura, N. Terasaka, Y. Goto,‡ K. Ogata, T. Sengoku, H. Onaka, and H. Suga.‡ De Novo Discovery of Thiopeptide Pseudo-natural Products Acting as Potent and Selective TNIK Kinase Inhibitors. J. Am. Chem. Soc. 2022, 144, 20332−20341.

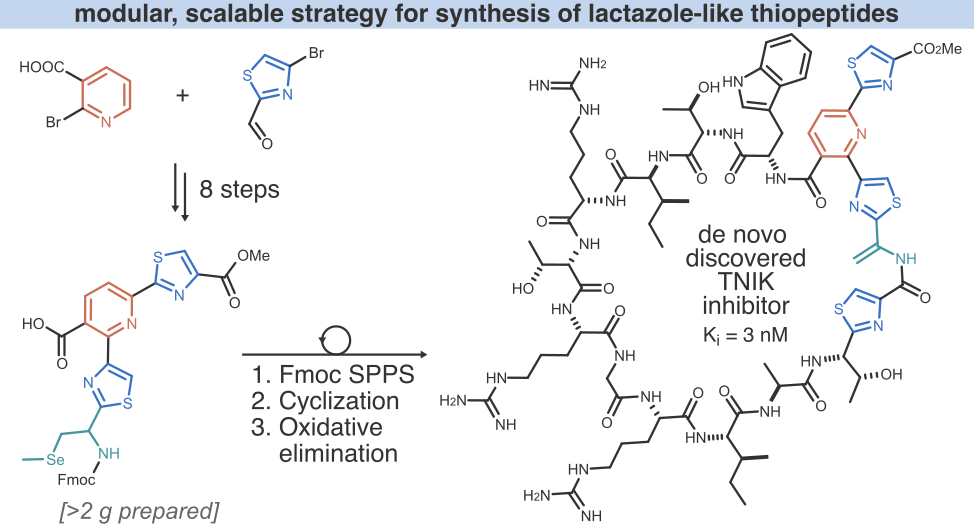
- Y. Zhang, A. Vinogradov,‡ J. Chang, Y. Goto, and H. Suga.‡ Solid-Phase-Based Synthesis of Lactazole-Like Thiopeptides. Org. Lett. 2022, 24, 7894−7899.
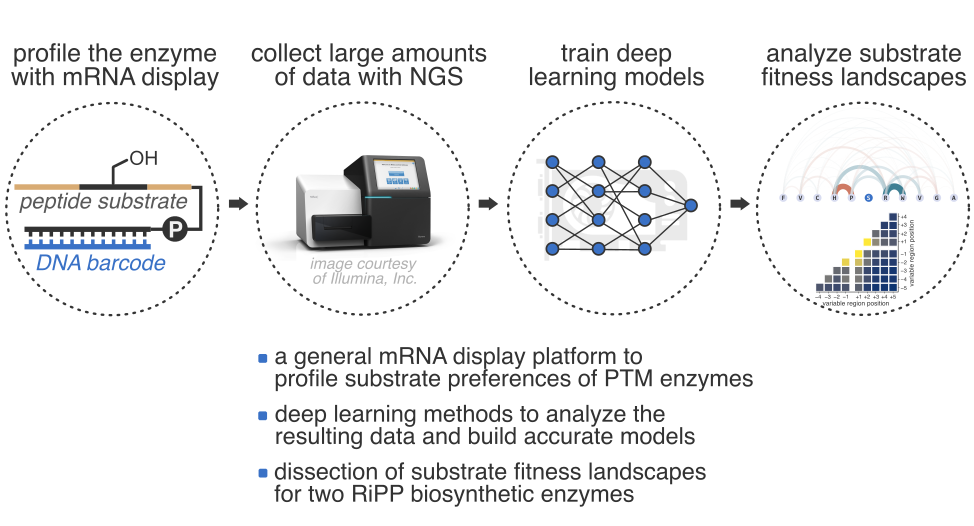
- A. Vinogradov,‡ J. Chang, H. Onaka, Y. Goto, and H. Suga.‡ Accurate Models of Substrate Preferences of Post-Translational Modification Enzymes from a Combination of mRNA Display and Deep Learning. ACS Cent. Sci. 2022, 8, 814−824.
- A. Vinogradov,‡ M. Nagano, Y. Goto, and H. Suga.‡ Site-Specific Nonenzymatic Peptide S/O-Glutamylation Reveals the Extent of Substrate Promiscuity in Glutamate Elimination Domains. J. Am. Chem. Soc. 2021, 143, 13358–13369.
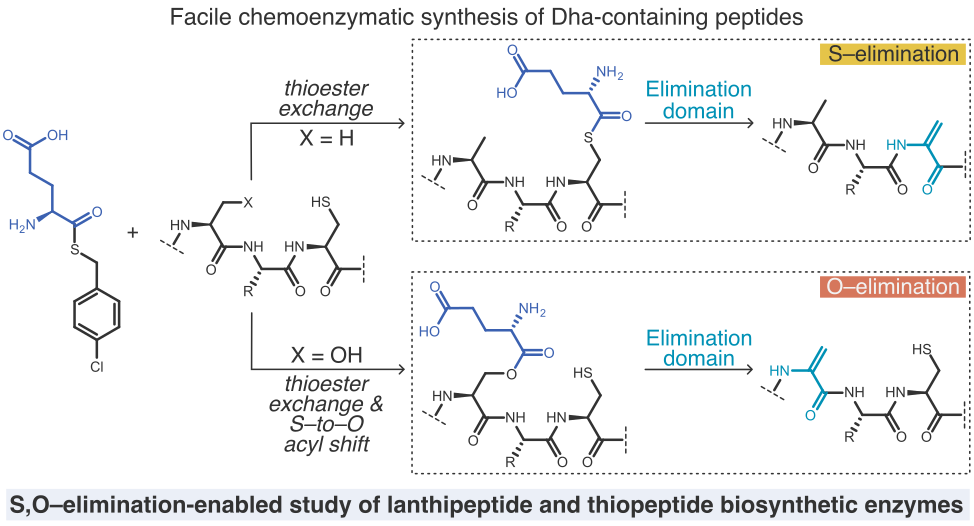
- A. Vinogradov,‡ E. Nagai, J. Chang, K. Narumi, H. Onaka,‡ Y. Goto,‡ and H. Suga.‡ Accurate Broadcasting of Substrate Fitness for Lactazole Biosynthetic Pathway from Reactivity-Profiling mRNA Display. J. Am. Chem. Soc. 2020, 142, 20329−20334.
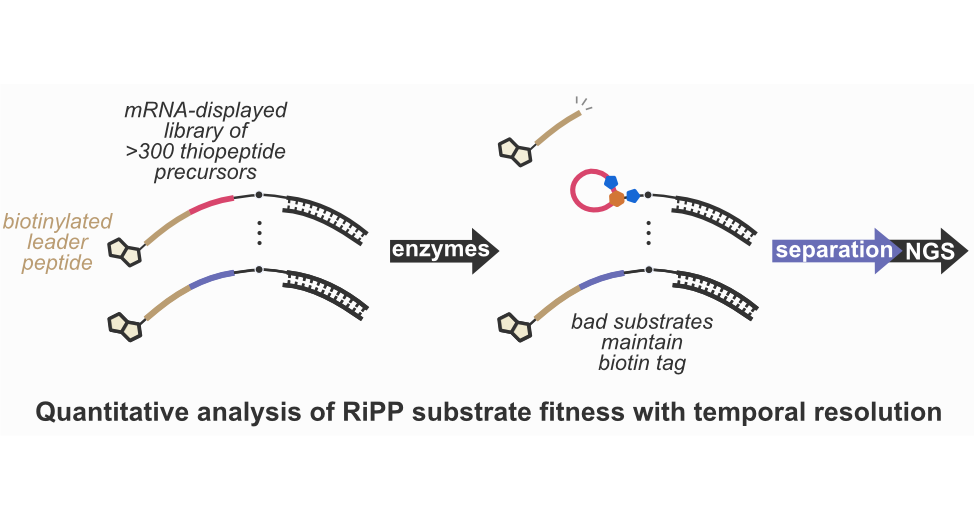
- A. Vinogradov, M. Shimomura, N. Kano, Y. Goto, H. Onaka, and H. Suga. ‡ Promiscuous Enzymes Cooperate at the Substrate Level En Route to Lactazole A. J. Am. Chem. Soc. 2020, 142, 13886–13897.
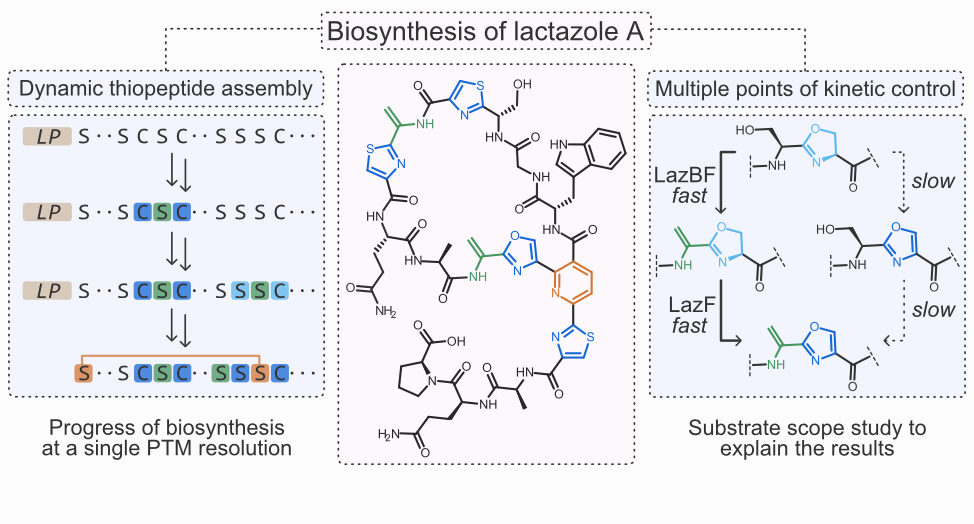
- A. Vinogradov,† M. Shimomura,† Y. Goto,‡ T. Ozaki, S. Asamizu, Y. Sugai, H. Suga,‡ and H. Onaka.‡ Minimal lactazole scaffold for in vitro thiopeptide bioengineering. Nat. Commun. 2020, 11, 2272.

- O. Bondarenko,‡ A. Vinogradov, A. Komarov, G. Karetnikov, N. Zyk, T. Holt, and A. Kutateladze. Access to 5-fluoroisoxazoles via the nitrosation of geminal bromo-fluoro arylcyclopropanes. Tetrahedron. 2019, 75, 2861–2865.

- S. Fleming, T. Bartges, A. Vinogradov, C. Kirkpatrick, Y. Goto, H. Suga, L. Hicks, and A. Bowers.‡ Flexizyme-Enabled Benchtop Biosynthesis of Thiopeptides. J. Am. Chem. Soc., 2019, 141, 758–762.

- Z. Gates,‡ A. Vinogradov, A. Quartararo, A. Bandyopadhyay, Z.-N. Choo, E. Evans, K. Halloran, A. Mijalis, S. Mong, M. Simon, E. Standley, E. Styduhar, S. Tasker, F. Touti, J. Weber, J. Wilson, T. Jamison, and B. Pentelute.‡ Xenoprotein engineering via synthetic libraries. Proc. Natl. Acad. Sci. U. S. A., 2018, 115, E5298–E5306.
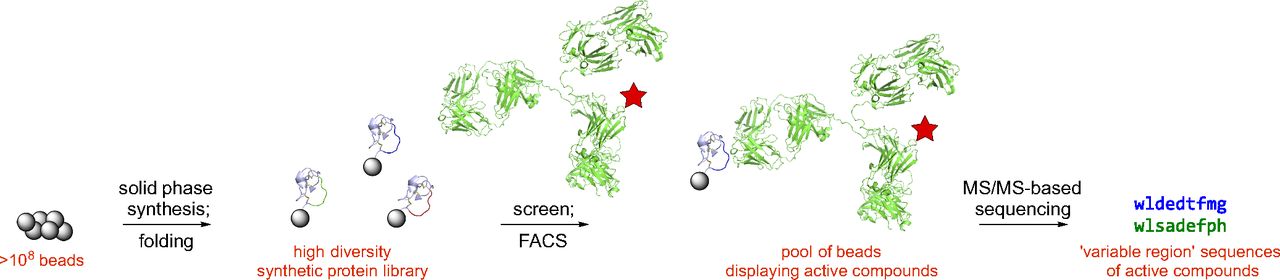
- C. Zhang, P. Dai, A. Vinogradov, Z. Gates, and B. Pentelute.‡ Site-Selective Cysteine–Cyclooctyne Conjugation. Angew. Chem. Int. Ed. 2018, 57, 6459–6463.
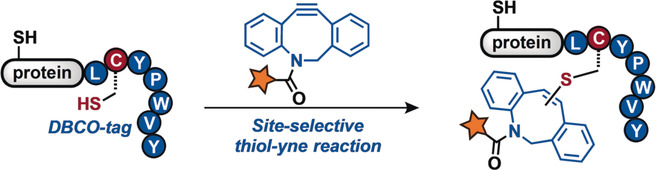
- A. Vinogradov,‡ Z. Gates, C. Zhang, A. Quartararo, K. Halloran, and B. Pentelute.‡ Library Design-Facilitated High-Throughput Sequencing of Synthetic Peptide Libraries. ACS Comb. Sci. 2017, 19, 694−701.
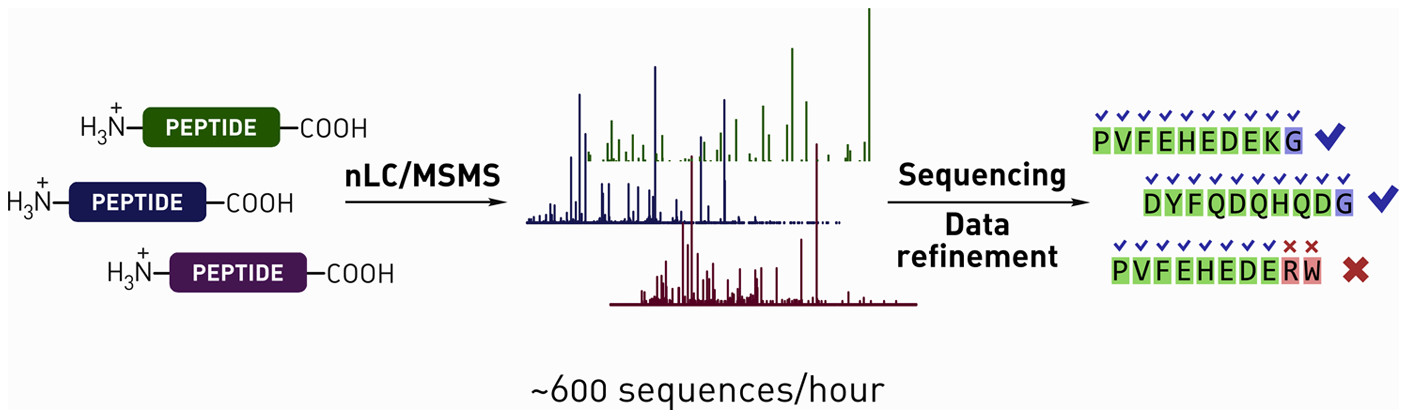
- M. Simon, Y. Maki, A. Vinogradov, C. Zhang, H. Yu, Y.-S. Lin, Y. Kajihara, and B. Pentelute.‡ D-amino acid scan of two small proteins. J. Am. Chem. Soc., 2016, 138, 12099−12111.

- A. Vinogradov, Z.-N. Choo, K. Totaro, and B. Pentelute.‡ Macrocyclization of Unprotected Peptide Isocyanates. Org. Lett. 2016, 18, 1226–1229.
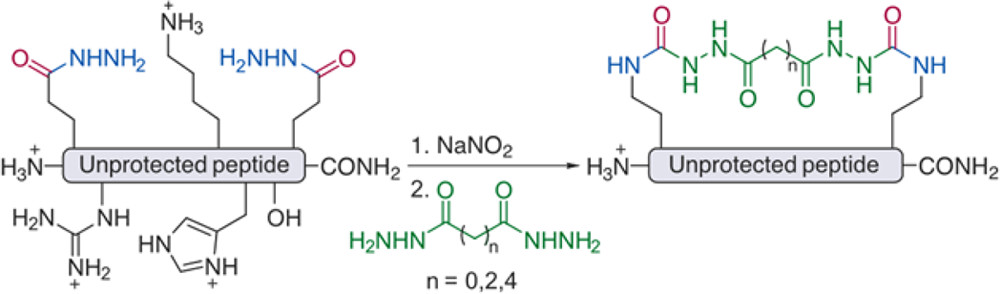
- A. Vinogradov, M. Simon, and B. Pentelute.‡ C‑Terminal Modification of Fully Unprotected Peptide Hydrazides via in Situ Generation of Isocyanates. Org. Lett. 2016, 18, 1222–1225.
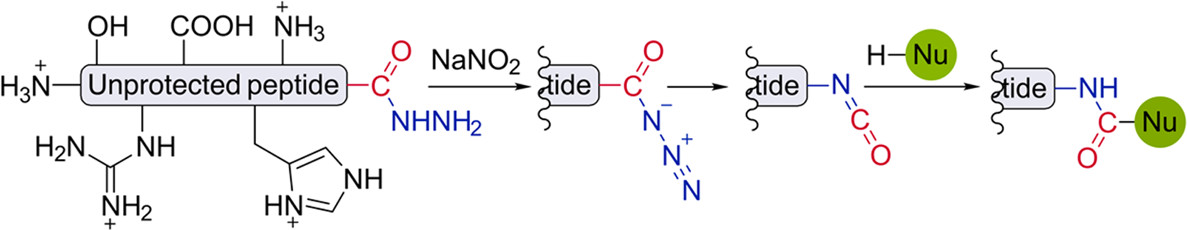
- O. Bondarenko,‡ A. Vinogradov, A. Komarov, A. Smirnov, and N. Zyk. Synthesis of 5-fluoro- and 5-bromoalkylisoxazoles via nitrosation of 1,1-dihalocyclopropanes with sulfur trioxide activated nitrosyl chloride. J. Fluor. Chem. 2016, 185, 201–205.

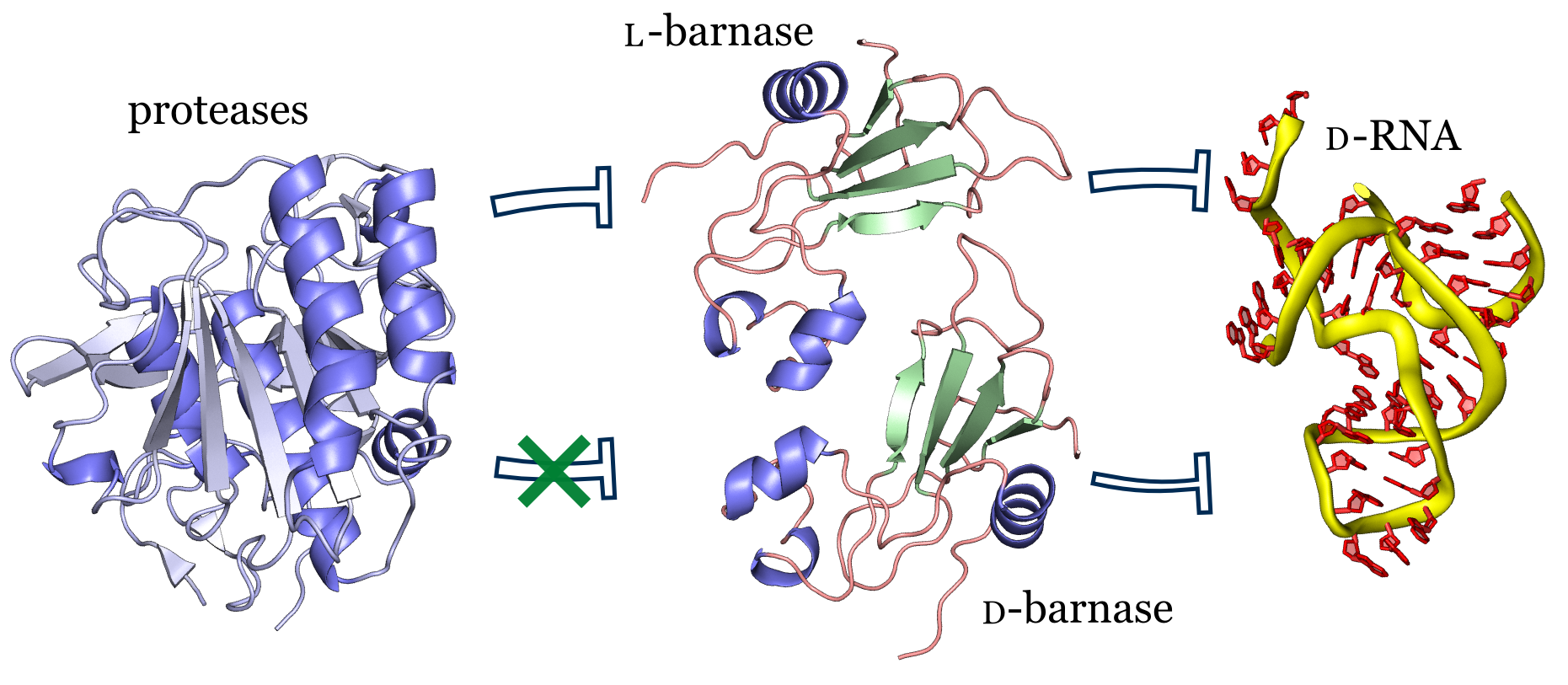
- A. Vinogradov, E. Evans, and B. Pentelute.‡ Total synthesis and biochemical characterization of mirror image barnase. Chem. Sci. 2015, 6, 2997–3002.
- O. Bondarenko,‡ A. Vinogradov, P. Danilov, S. Nikolaeva, A. Gavrilova, and N. Zyk. Nitrosation of 2-aryl-1,1-dibromocyclopropanes: synthesis of 3-aryl-5-bromoisoxazoles. Tetrahedron Lett. 2015, 56, 6577–6579.

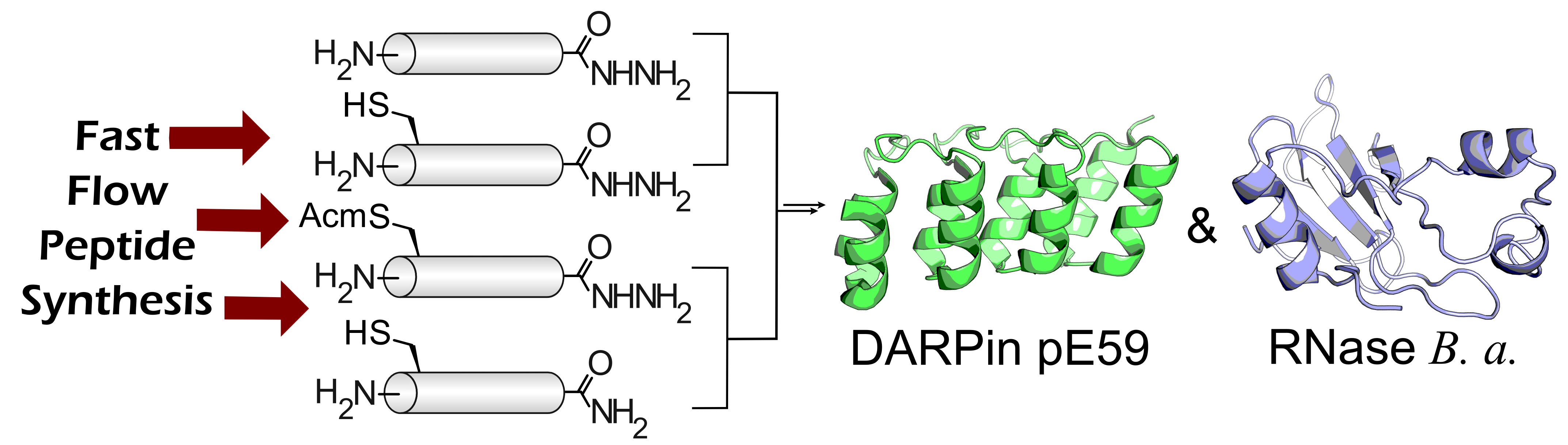
- S. Mong,† A. Vinogradov,† M. Simon, and B. Pentelute.‡ Rapid Total Synthesis of DARPin pE59 and Barnase. ChemBioChem 2014, 15, 721–733.
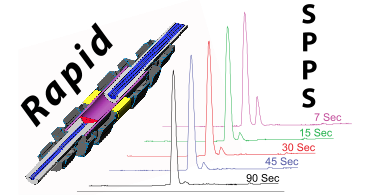
- M. Simon, P. Heider, A. Adamo, A. Vinogradov, S. Mong, X. Li, T. Berger, R. Policarpo, C. Zhang, Y. Zou, X. Liao, A. Spokoyny, K. Jensen, and B. Pentelute.‡ Rapid Flow-Based Peptide Synthesis. ChemBioChem 2014, 15, 713–720.
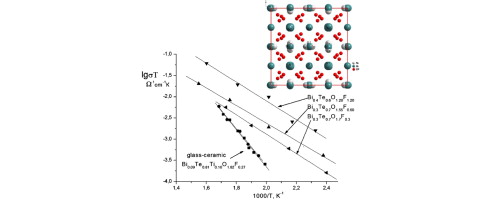
- V. Prituzhalov, E. Ardashnikova,‡ A. Vinogradov, V. Dolgikh, J.-J. Videau, E. Fargin, A. Abakumov, N. Tarakina, and G. Van Tendeloo. New anion-conducting solid solutions Bi1−xTex(O,F)2+δ (x > 0.5) and glass–ceramic materials on their base. J. Fluor. Chem. 2011, 132, 1110–1116.
Reviews
- A. Vinogradov‡ and H. Suga.‡ Introduction to Thiopeptides: Biological Activity, Biosynthesis, and Strategies for Functional Reprogramming. Cell Chem. Biol. 2020, 27, 1032–1051.
- A. Vinogradov, Y. Yin, and H. Suga.‡ Macrocyclic Peptides as Drug Candidates: Recent Progress and Remaining Challenges. J. Am. Chem. Soc. 2019, 141, 4167–4181.
Book chapters
- M. Simon, A. Mijalis, K. Totaro, D. Dunkelmann, A. Vinogradov, C. Zhang, Y. Maki, J. Wolfe, J. Wilson, A. Loas, and B. Pentelute.‡ Automated Fast Flow Peptide Synthesis, in Total Chemical Synthesis of Proteins, John Wiley & Sons, 2021, 17–58.
Link to chapter - R. van Neer, A. Vinogradov, and H. Suga.‡ RaPID Discovery of Macrocyclic Peptide Inhibitors of Protein–Protein Interactions, in Inhibitors of Protein–Protein Interactions, Royal Society of Chemistry, 2020, 232–246.
Link to chapter